[00:01]: [Taltz logo appears on the screen]
[VO Narration]: This video will show you how to properly administer Taltz…
[00:06]: [Dosing information appears on the screen]
[VO Narration]: …using the autoinjector.
[Caption]: Taltz Logo
Presents
Using your Taltz autoinjector (80 mg/mL)
Please see safety information throughout this video and the Purpose and Safety Summary
below the video.
[00:07]: [Dosing information appears on the screen]
[VO narration: Please see safety information throughout this video…
[Caption]: Taltz Logo
Presents
Using your Taltz autoinjector (80 mg/mL)
Please see safety information throughout this video and the Purpose and Safety Summary
below the video.
[00:09]: [Dosing information appears on the screen]
[VO narration]: …and the Purpose and Safety Summary below the video.
[Caption]: Taltz Logo
Presents
Using your Taltz autoinjector (80 mg/mL)
Please see safety information throughout this video and the Purpose and Safety Summary
below the video.
[00:14]: [Dosing information appears on the screen]
[VO narration]: It is important to read the Instructions for Use before injecting.
[Caption]: Taltz Logo
19291_ELI_IPO_L10212 IFU Video Update: Autoinjector
Presents
Using your Taltz autoinjector (80 mg/mL)
Read the Instructions for Use before injecting.
[00:18]: [Dosing information appears on the screen]
[VO narration]: Do not use until you have been shown how to inject Taltz…
[Caption]: Taltz Logo
Presents
Using your Taltz autoinjector (80 mg/mL)
Do not use until you have been shown how to inject Taltz by your healthcare provider. If you
are a parent or caregiver giving an injection to someone else, be sure to carefully read and
understand the Instructions for Use before injecting.
[00:23]: [Injection steps appear on the screen]
[VO narration]: …by your healthcare provider. If you are a parent or caregiver giving…
[Caption]: Taltz Logo
Do not use until you have been shown how to inject Taltz by your healthcare provider. If you
are a parent or caregiver giving an injection to someone else, be sure to carefully read and
understand the Instructions for Use before injecting.
[00:25]: [Injection steps appear on the screen]
[VO narration]: …an injection to someone else, be sure to carefully read and understand…
[Caption]: Taltz Logo
Do not use until you have been shown how to inject Taltz by your healthcare provider. If you
are a parent or caregiver giving an injection to someone else, be sure to carefully read and
understand the Instructions for Use before injecting.
[00:30]: [Injection steps appear on the screen]
19291_ELI_IPO_L10212 IFU Video Update: Autoinjector
[VO narration]: ...the Instructions for Use before injecting.
[Caption]: Taltz Logo
Do not use until you have been shown how to inject Taltz by your healthcare provider. If you
are a parent or caregiver giving an injection to someone else, be sure to carefully read and
understand the Instructions for Use before injecting.
[00:36]: [Man waking up]
[VO self talk]: It’s going to be a great weekend.
[Caption]: Actor portrayal.
[00:38]: [Man waking up]
[VO self talk]: Quality time on the beach with my family.
[Caption]: Actor portrayal.
[00:40]: [Man waking up]
[VO self talk]: Almost ready to head out. Sunscreen, bathing suit, and…
[00:44]: [Man checking his phone]
[VO self talk]: …Oh right!
[Caption]:
TO DO
- Pack car
- Water plants
- Taltz injection
[00:45]: [Man checking his phone]
[VO self talk]: It’s my next monthly Taltz injection day!
[Caption]:
TO DO
19291_ELI_IPO_L10212 IFU Video Update: Autoinjector
[00:49]: [Man getting off his bed]
[VO self talk]: I wasn’t sure what to expect the first time I used the autoinjector at home.
[Caption]:
EASY TO USE
At first use, over 94% of adult patients with moderate to severe plaque psoriasis agreed that
the Taltz autoinjector was “easy to use” and were confident in their ability to use it.
[00:53]: [Man looking out of the window]
[VO self talk]: But now that I know how, I think it’s easy to use.
[Caption]:
EASY TO USE
At first use, over 94% of adult patients with moderate to severe plaque psoriasis agreed that
the Taltz autoinjector was “easy to use” and were confident in their ability to use it.
[00:57]: [Man packing a bag]
[VO self talk]: Making Taltz part of my routine was simple…
[Caption]:
EASY TO USE
At first use, over 94% of adult patients with moderate to severe plaque psoriasis agreed that
the Taltz autoinjector was “easy to use” and were confident in their ability to use it.
Your initial dosing schedule may differ based on your diagnosis. Talk to your doctor.
[01:00]: [Man walking out of his room]
[VO self talk]: …with one injection every 4 weeks.
[Caption]:
CAREGIVER
If you are a caregiver giving an injection to someone else, be sure to carefully read and
understand the Instructions for Use before injecting.
Your initial dosing schedule may differ based on your diagnosis. Talk to your doctor.
19291_ELI_IPO_L10212 IFU Video Update: Autoinjector
[01:03]: [Man talking to a kid]
[VO self Talk]: Do not use until you have been shown…
[Caption]:
CAREGIVER
If you are a caregiver giving an injection to someone else, be sure to carefully read and
understand the Instructions for Use before injecting.
[01.06]: [Kid packing his bag]
[VO self talk]: …how to inject Taltz by your healthcare provider.
[Caption]:
CAREGIVER
If you are a caregiver giving an injection to someone else, be sure to carefully read and
understand the Instructions for Use before injecting.
[01.10]: [Man and his partner are loading bags in the trunk]
[VO self talk]: In just 3 injection steps I am done!
[Caption]:
3 INJECTION STEPS
- Uncap the autoinjector
- Place and unlock
- Press and hold
Carefully read the Instructions for Use that came with the device before injecting. If you have
vision or hearing problems, do not use the Taltz autoinjector without help from a caregiver.
[01:11]: [Man and his partner are loading bags in the trunk]
[VO self talk]: That’s easy to stick to!
[Caption]:
3 INJECTION STEPS
- Uncap the autoinjector
- Place and unlock
- Press and hold
19291_ELI_IPO_L10212 IFU Video Update: Autoinjector
Carefully read the Instructions for Use that came with the device before injecting. If you have
vision or hearing problems, do not use the Taltz autoinjector without help from a caregiver.
[01.17]: [Man checking his phone]
[VO self talk]: On my next injection day, when we’re not busy packing,…
Caption: CHOOSE YOUR INJECTION SITE
Alternate injection site each time. You can use the same area of your body but choose a
different spot in that area.
[01.19]: [Man checking his phone]
[VO self talk]: …I will ask my wife to help me so I can inject the back of my upper arm.
[Caption]: CHOOSE YOUR INJECTION SITE
Alternate injection site each time. You can use the same area of your body but choose a
different spot in that area.
[01:23]: [Man looking at his arm]
[VO self talk]: Though I know I can also inject my thigh like I did last time.
[Caption]: CHOOSE YOUR INJECTION SITE
Avoid areas with psoriasis, scars, bruises, or injecting too close to your belly button.
[01:26]: [Man holding his belly and thinking about his injection site]
[VO self Talk]: I think today I will inject Taltz in my belly.
[Caption]: CHOOSE YOUR INJECTION SITE
Avoid areas with psoriasis, scars, bruises, or injecting too close to your belly button.
[01:31]: [Man opening the refrigerator]
[VO self talk]: I keep Taltz in my refrigerator and take it out 30 minutes…
[Caption]: KEEP REFRIGERATED
Keep Taltz in your refrigerator. Do not freeze, microwave, or run it under hot water. Keep out
of direct sunlight. Do not shake.
[01:34]: [Man removing the Taltz autoinjector from the refrigerator]
[VO self talk]: …before injecting so it can warm up to room temperature.
19291_ELI_IPO_L10212 IFU Video Update: Autoinjector
[Caption]: KEEP REFRIGERATED
Keep Taltz in your refrigerator. Do not freeze, microwave, or run it under hot water. Keep out
of direct sunlight. Do not shake.
[01:37]: [Autoinjector carton is on the table]
[VO self talk]: But it’s good to know that I can keep it out in its original box…
[Caption]: KEEP CAP ON
Keep the cap on until you are ready to inject. Once Taltz has been stored at room temperature,
do not return it to the refrigerator.
[01:40]
[VO self talk]: …for up to 5 days away from direct light.
[Caption]: KEEP CAP ON
Keep the cap on until you are ready to inject. Once Taltz has been stored at room temperature,
do not return it to the refrigerator.
[Descriptive clue]: Autoinjector is on the table.
[01:47]: [Man washing his hands]
[VO self talk]: First, I need to wash my hands.
[Caption]: GET READY
Wash your hands.
[01:50]: [Man inspecting the autoinjector]
[VO self talk]: And make sure it hasn’t expired.
[Caption]: CHECK YOUR AUTOINJECTOR
Make sure you have the right medicine and that it has not expired. Check that it is not
damaged.
[01:54]: [Man inspecting the autoinjector]
[VO self talk]: Ok nothing is broken, and I don’t see anything different…
Caption: CHECK YOUR AUTOINJECTOR
Inspect the medicine. It should be clear to slightly yellow. Make sure it’s not cloudy or has
particles in it, and make sure the medicine is not frozen.
19291_ELI_IPO_L10212 IFU Video Update: Autoinjector
[01:56]: [Man inspecting the autoinjector]
[VO self talk]: …or floating around in the liquid.
[Caption]: CHECK YOUR AUTOINJECTOR
Inspect the medicine. It should be clear to slightly yellow. Make sure it’s not cloudy or has
particles in it, and make sure the medicine is not frozen.
[02:04]: [Man cleaning the injection site]
[VO self talk]: First, I’ll clean the injection site with an alcohol wipe.
[Caption]: PREPARE YOUR SKIN
Clean your injection site.
[02:09]: [Man holding the autoinjector]
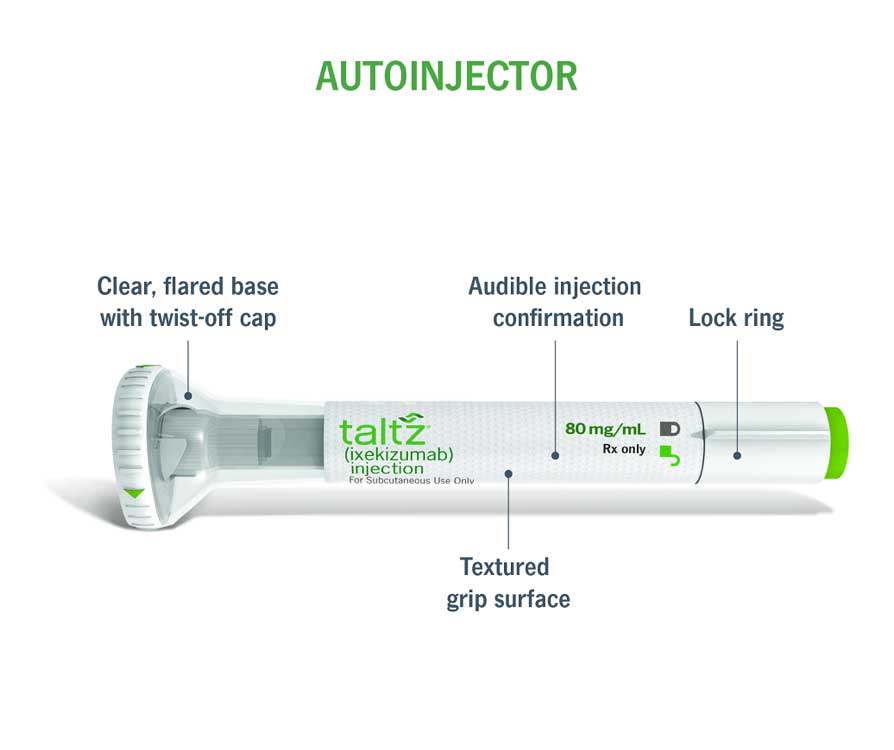
[VO self talk]: I’ll check that the lock ring is in the lock position.
[Descriptive clue]: The autoinjector is being held between the thumb and fingers to inspect the
lock ring.
[02:12]: [Uncapping of the syringe]
[VO self talk]: Now I’ll remove the cap…
[Caption]: STEP 01
Uncap the autoinjector
[Descriptive clue]: The device is being held between the thumb and fingers while being
uncapped with the other hand.
[02:16]: [Man holding the autoinjector against his belly]
[VO self talk]: …and hold the autoinjector firmly against my belly.
[Caption]: STEP 02
Place and unlock
[02:17]: [Man holding the autoinjector against his belly]
[VO self talk]: Now I’ll unlock it.
[Caption]: STEP 02
Place and unlock
19291_ELI_IPO_L10212 IFU Video Update: Autoinjector
[02:19]: [Man is about to press the green button on the autoinjector]
[VO self talk]: All I need to do is press and hold the green button until I hear a click.
[Caption]: STEP 02
Place and unlock
[02:22]: [Man is pressing the green button on the autoinjector]
[SFX]: (Click sound)
[Caption]: STEP 03
Press and hold
[02:23]: [Man is pressing the green button on the autoinjector]
[VO self talk]: That lets me know the injection has started.
[Caption]: STEP 03
Press and hold
[02:25]: [Man is pressing the green button on the autoinjector]
[VO self talk]: I love that I don’t have to see the needle!
[Caption]: STEP 03
Press and hold
[02:27]: [Man is pressing the green button on the autoinjector]
[VO self talk]: I’ll just continue to hold the button until I hear a second click.
[Caption]: STEP 03
Press and hold
[02:31]: [Man is pressing the green button on the autoinjector]
[SFX]: (Click sound)
[02:32]: [Man is pressing the green button on the autoinjector while looking down on the
injection site.]
[VO self talk]: And there it is!
[Caption]: FINISH
19291_ELI_IPO_L10212 IFU Video Update: Autoinjector
Do not put the base cap back on the autoinjector before throwing it away.
[02:34]: [Man removes the autoinjector slowly]
[VO self talk]: The second click lets me know I’m all done.
[Caption]: FINISH
Do not put the base cap back on the autoinjector before throwing it away.
[02:37]: [Man is cleaning his injection site]
[VO self talk]: I know my injection is complete because I can see the plunger.
[Caption]: FINISH
Do not put the base cap back on the autoinjector before throwing it away.
[02:41]: [Man is holding the alcohol swap against his belly]
[VO self talk]: I don’t want to leave the autoinjector lying around,…
[Caption]: DISPOSE OF PROPERLY
Do not throw away autoinjectors in your household trash. Do not recycle your used sharps
disposal container.
[02:43]: [Man is holding the alcohol swap against his belly]
[VO self talk]: …and I can’t just throw this in the regular trash.
[Caption]: DISPOSE OF PROPERLY
Do not throw away autoinjectors in your household trash. Do not recycle your used sharps
disposal container.
[02:46]: [Man is wiping the injection site]
[VO self talk]: I will dispose of it right after use in the free sharps container that…
[Caption]: DISPOSE OF PROPERLY
Keep sharps container away from children and pets.
[02:49]: [Man is disposing the autoinjector in the sharps container]
[VO self talk]: …Taltz Together™ provided.
[Caption]: Some areas have special rules about what to do with filled sharps containers, so be
sure to ask your healthcare provider about it.
19291_ELI_IPO_L10212 IFU Video Update: Autoinjector
[02:52]: [Man is closing the sharps container]
[VO self talk]: That didn’t take long…
[Caption]:
3 INJECTION STEPS
- Uncap the autoinjector
- Place and unlock
- Press and hold
Some areas have special rules about what to do with filled sharps containers, so be sure to ask
your healthcare provider about it.
[02:56]: [Man is grabbing the box with his partner]
[VO self talk]: …and now I can enjoy the weekend!
[Caption]: Taltz Together Logo
Taltz Together™ can also provide additional support with over-the-phone training by a
registered nurse.
[02:58]: [Man is packing with his partner]
[VO self talk]: Swimsuit, sunshine, and family.
[Caption]: Taltz Together Logo
Taltz Together™ can also provide additional support with over-the-phone training by a
registered nurse.
[03:00]: [Man is heading out of his house]
[VO self talk]: It’s going to be a great weekend.
[Caption]: Taltz Together Logo
Taltz Together™ can also provide additional support with over-the-phone training by a
registered nurse.
[03:03]: [Man is heading out of his house and his family joins him]
[VO narration]: Do not use if you are allergic to Taltz. Before starting, you should be…
[Caption]: Refer to the full Instructions for Use that came with the device.
19291_ELI_IPO_L10212 IFU Video Update: Autoinjector
[03:08]: [Man is heading out of his house and his family joins him]
[VO narration]: …checked for tuberculosis. Taltz may increase your risk of infections…
[Caption]: Refer to the full Instructions for Use that came with the device.
[03:13]: [Man is loading stuff in his car]
[VO narration]: …and lower your ability to fight them.
[Caption]: Refer to the full Instructions for Use that came with the device.
[03:15]: [Woman is opening the car door]
[VO narration]: Tell your doctor if you are being treated for an infection,…
[Caption]: CALL
Call 1-844-TALTZ-NOW (1-844-825-8966) or come back to this video anytime at Taltz.com
[03:19]: [Family is getting in the car]
[VO narration]: …or if you have received a vaccine or plan to.
[Caption]: CALL
Call 1-844-TALTZ-NOW (1-844-825-8966) or come back to this video anytime at Taltz.com
[03:21]: [Family is getting ready to leave]
[VO narration]: Call your doctor right away if you have symptoms of an infection,…
[Caption]: CALL
Call 1-844-TALTZ-NOW (1-844-825-8966) or come back to this video anytime at Taltz.com
[03:25]: [Man’s hand is on the gear lever]
[VO narration]: …such as fever, sweats, chills, muscle aches, or cough.
[Caption]: CALL
Call 1-844-TALTZ-NOW (1-844-825-8966) or come back to this video anytime at Taltz.com
[03:29]: [Man is looking back at the rear window]
[VO narration]: Inflammatory bowel disease (Crohn's disease or ulcerative colitis)…
19291_ELI_IPO_L10212 IFU Video Update: Autoinjector
[03:34]: [Man and his partner are holding hands]
[VO narration]: …can happen with Taltz, including worsening of symptoms.
[03:38]: [Family exiting the driveway]
[VO narration]: Serious allergic reactions can occur.
[Caption]: Please see safety information and the Purpose and Safety Summary below the
video. For more information visit Taltz.com
[03:48]: [Taltz logo and indication statement appear on the screen]
[VO narration]: Taltz is an injectable medicine used to treat adults with active psoriatic…
[Caption]: Taltz logo
Taltz is an injectable medicine used to treat adults with active psoriatic arthritis. It is also used
to treat adults with active ankylosing spondylitis and active non-radiographic axial
spondyloarthritis with objective signs of inflammation, as well as people 6 years of age and
older with moderate to severe plaque psoriasis who may benefit from taking injections or pills
(systemic therapy) or treatment using ultraviolet or UV light (phototherapy).
[03:53]: [Taltz logo and indication statement appear on the screen]
[VO narration]: …arthritis. It is also used to treat adults with active ankylosing spondylitis…
[Caption]: Taltz logo
Taltz is an injectable medicine used to treat adults with active psoriatic arthritis. It is also used
to treat adults with active ankylosing spondylitis and active non-radiographic axial
spondyloarthritis with objective signs of inflammation, as well as people 6 years of age and
older with moderate to severe plaque psoriasis who may benefit from taking injections or pills
(systemic therapy) or treatment using ultraviolet or UV light (phototherapy).
[03:58]: [Taltz logo and indication statement appear on the screen]
[VO narration]: …and active non-radiographic axial spondyloarthritis with objective signs…
[Caption]: Taltz logo
Taltz is an injectable medicine used to treat adults with active psoriatic arthritis. It is also used
to treat adults with active ankylosing spondylitis and active non-radiographic axial
spondyloarthritis with objective signs of inflammation, as well as people 6 years of age and
older with moderate to severe plaque psoriasis who may benefit from taking injections or pills
(systemic therapy) or treatment using ultraviolet or UV light (phototherapy).
19291_ELI_IPO_L10212 IFU Video Update: Autoinjector
[04:03]: [Taltz logo and indication statement appear on the screen]
[VO narration]: …of inflammation, as well as people 6 years of age and older with…
[Caption]: Taltz logo
Taltz is an injectable medicine used to treat adults with active psoriatic arthritis. It is also used
to treat adults with active ankylosing spondylitis and active non-radiographic axial
spondyloarthritis with objective signs of inflammation, as well as people 6 years of age and
older with moderate to severe plaque psoriasis who may benefit from taking injections or pills
(systemic therapy) or treatment using ultraviolet or UV light (phototherapy).
[04.07]: [Taltz logo and indication statement appear on the screen]
[VO narration]: …moderate to severe plaque psoriasis who may benefit from taking…
[Caption]: Taltz logo
Taltz is an injectable medicine used to treat adults with active psoriatic arthritis. It is also used
to treat adults with active ankylosing spondylitis and active non-radiographic axial
spondyloarthritis with objective signs of inflammation, as well as people 6 years of age and
older with moderate to severe plaque psoriasis who may benefit from taking injections or pills
(systemic therapy) or treatment using ultraviolet or UV light (phototherapy).
[04:11]: [Taltz logo and indication statement appear on the screen]
[VO narration]: …injections or pills (systemic therapy) or treatment using…
[Caption]: Taltz logo
Taltz is an injectable medicine used to treat adults with active psoriatic arthritis. It is also used
to treat adults with active ankylosing spondylitis and active non-radiographic axial
spondyloarthritis with objective signs of inflammation, as well as people 6 years of age and
older with moderate to severe plaque psoriasis who may benefit from taking injections or pills
(systemic therapy) or treatment using ultraviolet or UV light (phototherapy).
[04:15]: [Taltz logo and indication statement appear on the screen]
[VO narration]: …ultraviolet or UV light (phototherapy).
[Caption]: Taltz logo
Taltz is an injectable medicine used to treat adults with active psoriatic arthritis. It is also used
to treat adults with active ankylosing spondylitis and active non-radiographic axial
spondyloarthritis with objective signs of inflammation, as well as people 6 years of age and
19291_ELI_IPO_L10212 IFU Video Update: Autoinjector
older with moderate to severe plaque psoriasis who may benefit from taking injections or pills
(systemic therapy) or treatment using ultraviolet or UV light (phototherapy).
[04:21]: [Taltz logo and headline appear on the screen]
[VO narration]: Uncover the possibilities with Taltz!
[Caption]:
Taltz logo
Uncover the possibilities with Taltz
[04:24]: [Taltz logo, Lilly logo and headline appear on the screen]
[VO narration]: Uncover the possibilities with Taltz!
[Caption]:
Taltz logo
Lilly Logo
Uncover the possibilities with Taltz
TaltzR is a registered trademark owned or licensed by Eli Lilly and Company, its subsidiaries or
affiliates.
Taltz Together™ is a trademark of Eli Lilly and Company.
PP-IX-US-5222 08/2022 c Lilly USA, LLC 2022. All rights reserved.