00:00-00:42
[Taltz logo with dosage on screen in lower right corner.]
Caption:
INDICATIONS
Taltz is indicated for adults with active psoriatic arthritis (PsA), for adults with active ankylosing spondylitis (AS), and for adults with active non-radiographic axial spondyloarthritis (nr-axSpA) with objective signs of inflammation. Taltz is also indicated for patients aged 6 years or older with moderate-to-severe plaque psoriasis (PsO) who are candidates for systemic therapy or phototherapy.
CONTRAINDICATIONS
Taltz is contraindicated in patients with a previous serious hypersensitivity reaction, such as anaphylaxis, to ixekizumab or to any of the excipients. Full Important Safety Information is available at the conclusion of the video.
Narrator: INDICATIONS, Taltz is indicated for adults with active psoriatic arthritis (PsA), for adults with active ankylosing spondylitis (AS), and for adults with active non-radiographic axial spondyloarthritis (nr-axSpA) with objective signs of inflammation. Taltz is also indicated for patients aged 6 years or older with moderate-to-severe plaque psoriasis (PsO) who are candidates for systemic therapy or phototherapy.
CONTRAINDICATIONS, Taltz is contraindicated in patients with a previous serious hypersensitivity reaction, such as anaphylaxis, to ixekizumab or to any of the excipients. Full Important Safety Information is available at the conclusion of the video.
00:43-00:57
[Drawing of patient appears on screen showing plaques on their arms and scalp, and their nails are discolored. Drawings highlighting scalp and nail psoriasis appear.]
Caption:
Recognizing predictors of psoriatic arthritis in plaque psoriasis patients
Narrator: Recognizing predictors of psoriatic arthritis in plaque psoriasis patients.
Patients with moderate-to-severe plaque psoriasis may struggle with the progression of their disease in a multitude of ways.
00:58-01:11
[Drawing of a hand highlighting inflammation in the joints appears. Pie graph highlighting 30% in red and 70% in blue appears. The drawing of a hand is connected to the red 30% section of the pie graph.]
Caption:
Psoriatic Arthritis
Up to 30% psoriasis patients will also go on to develop psoriatic arthritis2-4
Narrator:
Those struggling with psoriasis can develop psoriatic arthritis. In fact, up to 30% of patients with psoriasis will also go on to develop psoriatic arthritis.
01:12-1:41
[Drawing of hand is in the upper right corner. Four person icons appear under an icon of scalp psoriasis, indicating 4x greater risk. Three person icons appear under an icon of nail psoriasis, indicating 3x greater risk. Timeline representing six months appears. Drawing of a patient with plaques holding their hand replaces the timeline on screen. Drawing of a hand with inflamed joints appears.]
Caption:
4x greater risk2, 3x greater risk2
6-month delay
Narrator: Specifically, people with psoriasis of the scalp or nails are at 4x and 3x greater risk, respectively. Awareness of psoriatic arthritis predictors is imperative because even a 6-month diagnostic delay may result in irreversible joint damage to the patient and worsened long-term physical function. Luckily, Taltz can help alleviate symptoms of both plaque psoriasis and psoriatic arthritis.
1:42-2:04
[Captions animate to fill the screen.]
Caption:
TALTZ REGISTRATION TRIALS:
Multicenter, randomized, double-blind, placebo-controlled1,5
UNCOVER-1, UNCOVER-2, UNCOVER-3 FOR ADULTS WITH MODERATE-TO-SEVERE PLAQUE PSORIASIS
Co-primary efficacy endpoints at week 12
- Proportion of patients achieving PASI 75
- Proportion of patients with sPGA 0,1 with at least a 2-point improvement from baseline
SCREENING RANDOMIZATION
12-WEEK INDUCTION PERIOD
UNCOVER-1 (N=864), Taltz 80 mg every 2 weeks* (n=433), Placebo (n=431)
UNCOVER-2 (N=877), Taltz 80 mg every 2 weeks (n=351)*, Enbrel§ 50 mg twice weekly (n=358), Placebo (n=168)
UNCOVER-3 (N=960), Taltz 80 mg every 2 weeks* (n=385), Enbrel§ 50 mg twice weekly (n=382), Placebo (n=193)
Week 0, Week 12
RE-RANDOMIZATION
At Week 12: Taltz Responders (sPGA 0,1) Re-Randomized to Taltz Q4W (n=221) and PBO (n=211)
Taltz 80 mg every 4 weeks, UNCOVER-1 (n=119)†, UNCOVER-2 (n=102)†
Placebo every 4 weeks, UNCOVER-1 (n=117), UNCOVER-2 (n=94)
OPEN-LABEL, LONG-TERM EXTENSION PERIOD
Taltz 80 mg every 4 weeks (n=362)
At Week 12: ALL patients went to Q4W Taltz
Week 60, Week 264
*Taltz-treated patients received a 160 mg starting dose
†Intent-to-Treat population: patients RANDOMIZED to receive 160 mg starting dose, 80 mg every 2 weeks for 12 weeks, and 80 mg every 4 weeks in the maintenance period.
§US-approved etanercept
PASI=Psoriasis Area Severity Index; PBO=placebo; Q4W=every 4 weeks; sPGA=static Physician Global Assessment.
Narrator: The designs of the Taltz registration trials in adults with moderate-to-severe plaque psoriasis are shown here. The co-primary efficacy endpoints of the trials were the proportion of patients achieving PASI 75 and proportion of patients with an sPGA of 0,1 and at least a 2-point improvement from baseline at Week 12.
2:05-2:26
[Captions animate to fill the screen.]
Caption:
TALTZ REGISTRATION TRIALS:
Multicenter, randomized, double-blind, placebo-controlled1
UNCOVER-1, UNCOVER-2, UNCOVER-3 FOR ADULTS WITH MODERATE-TO-SEVERE PLAQUE PSORIASIS
Results at Week 12, NRI, PASI 75, sPGA 0,1
UNCOVER-1 Taltz (80 mg Q2W); n=433, 89%, 82%
Placebo; n=431, 4%, 3%
UNCOVER-2 Taltz (80 mg Q2W); n=351, 90%, 83%
Placebo; n=168, 2%, 2%
UNCOVER-3 Taltz (80 mg Q2W); n=385, 87%, 81%
Placebo; n=193, 7%, 7%
In the maintenance period of UNCOVER-1 and UNCOVER-2, 75% of patients receiving Taltz who achieved sPGA 0,1 at Week 12 maintained that response at Week 60 (n=181*) compared with 7% of patients receiving placebo (n=203*) (NRI analysis).1
*Evaluable patients at Week 60.
NRI=nonresponder imputation; PASI=Psoriasis Area Severity Index; Q2W=every 2 weeks; sPGA=static Physician Global Assessment.
Narrator: In UNCOVER trials, Taltz was shown to be superior to placebo in achieving PASI 75 and sPGA 0,1 at 12 weeks after starting treatment. In UNCOVER-1 and UNCOVER-2, of those who achieved sPGA 0,1 at Week 12, 75% maintained that response at Week 60.
2:27-2:43
[Captions animate to fill the screen.]
Caption:
Post-hoc subgroup analyses of patients with scalp psoriasis
Induction period and open-label extension
UNCOVER-3: MEAN PERCENT IMPROVEMENT FROM BASELINE IN PSSI AT WEEKS 12 AND 60, MMRM6
FOR ADULTS WITH MODERATE-TO-SEVERE PLAQUE PSORIASIS
MEAN CHANGE IN PSSI (%)
Induction Period WEEK 0, 2, 4, 8, 12
Taltz 80 mg every 2 weeks (induction) (n=185); then every 4 weeks (maintenance), 96%,
Mean score 1 (SD=4), Baseline score 30 (SD=11)
Placebo for 12 weeks (n=82), 23%, Mean score 22 (SD=13), Baseline score 29 (SD=10)
Open-Label Extension Period WEEK 12, 16, 20, 24, 28, 32, 36, 40, 44, 48, 52, 56, 60
Taltz 80 mg every 2 weeks, 95%, Mean score 1 (SD=3)
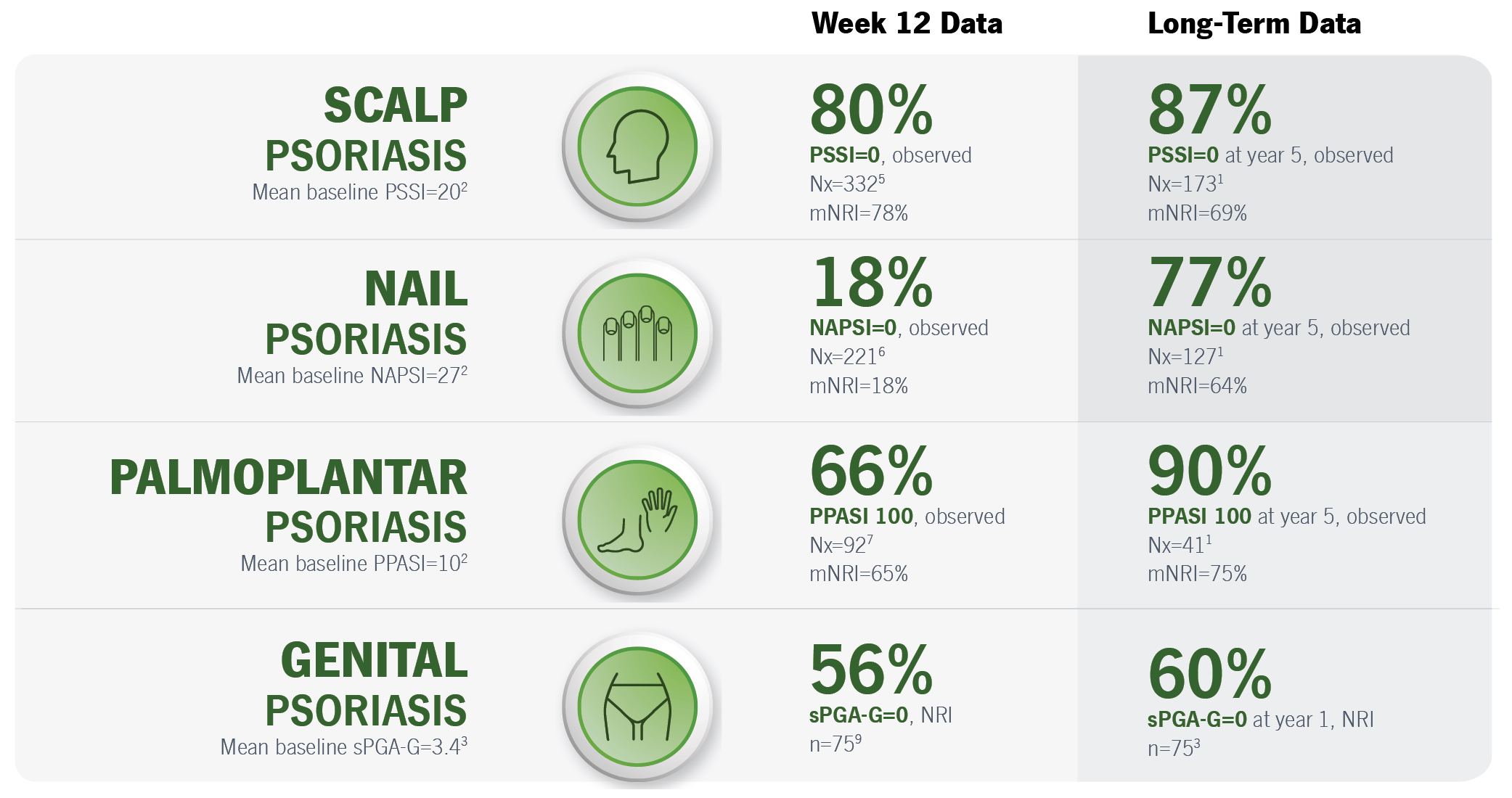
Subgroup analyses presented are post hoc. The UNCOVER studies were not powered for these subgroup analyses, nor were analyses error-controlled. Therefore, treatment differences observed in these subgroups cannot be regarded as statistically significant. Baseline severity was not specified in the inclusion criteria.
*A subset of patients from UNCOVER-3 with moderate to severe plaque psoriasis and scalp psoriasis (defined as PSSI score ≥15 and scalp involvement ≥30%) at baseline.6
PSSI score range 0-72.7
A positive percent change in PSSI represents a decrease in PSSI score. ADDITIONAL RESULTS: In UNCOVER-1 (Taltz 80 mg every 2 weeks n=210; placebo n=215), patients receiving Taltz achieved a mean PSSI of 2 (SD=7) (a 93% reduction from the mean Taltz baseline score of 32 (SD=12)) at Week 12. Patients receiving placebo achieved a mean PSSI of 27 (SD=16) (a 15% reduction from the mean baseline score of 32 (SD=14)). In UNCOVER-2 (Taltz 80 mg every 2 weeks n=144; placebo n=77), patients receiving Taltz achieved a mean PSSI of 2 (SD=5) (a 95% reduction from the mean Taltz baseline score of 32 (SD=13)) at Week 12. Patients receiving placebo achieved a mean PSSI of 24 (SD=14) (a 25% reduction from the mean baseline score of 33 (SD=12)).6
MMRM=mixed model of repeated measures; PSSI=Psoriasis Scalp Severity Index; SD=standard deviation.
Narrator: Taltz has been shown to improve scalp psoriasis from baseline, as early as 12 weeks following treatment initiation, with results sustained until Week 60. Shown is a post-hoc, subgroup analysis of UNCOVER-3.
2:44-2:59
[Captions animate to fill the screen.]
Caption:
Post-hoc subgroup analyses of patients with nail psoriasis
Induction period and open-label extension
UNCOVER-3: MEAN PERCENT IMPROVEMENT FROM BASELINE IN NAPSI AT WEEKS 12 and 60, MMRM6
FOR ADULTS WITH MODERATE-TO-SEVERE PLAQUE PSORIASIS
MEAN CHANGE IN NAPSI (%)
Induction Period WEEK 0, 2, 4, 8, 12 Taltz 80 mg every 2 weeks (induction) (n=138); then every 4 weeks (maintenance), 39%, Mean score 24 (SD=15), Baseline score 39 (SD=16)
Placebo for 12 weeks (n=71), -5%, Mean score 37 (SD=20), Baseline score 37 (SD=17)
Open-Label Extension Period WEEK 12, 16, 20, 24, 28, 32, 36, 40, 44, 48, 52, 56, 60
Taltz 80 mg every 2 weeks, 86%, Mean score 5 (SD=10)
Subgroup analyses presented are post hoc. The UNCOVER studies were not powered for these subgroup analyses, nor were analyses error-controlled. Therefore, treatment differences observed in these subgroups cannot be regarded as statistically significant. Baseline severity was not specified in the inclusion criteria.
*A subset of patients from UNCOVER-3 with moderate to severe plaque psoriasis who had nail psoriasis (defined as NAPSI score ≥16 with ≥4 fingernails involved) at baseline.8
NAPSI score range 0-80. Only patients’ fingernails were observed.9
A positive percent change in NAPSI represents a decrease from baseline in mean NAPSI. The mean number of fingernails involved at baseline was 9.2 for Taltz and 9.1 for placebo.8,9
ADDITIONAL RESULTS: In UNCOVER-1 (Taltz 80 mg every 2 weeks n=172; placebo n=168),
patients receiving Taltz achieved a mean NAPSI of 25 (SD=18) (a 33% reduction from the mean Taltz baseline score of 36 (SD=16)) at Week 12. Patients receiving placebo achieved a mean NAPSI of 40 (SD=17) at Week 12 (a 4% increase from the mean placebo baseline score of 39 (SD=16)). In UNCOVER-2 (Taltz n=131; placebo n=72), patients receiving Taltz achieved a mean NAPSI of 26 (SD=18) at Week 12 (a 34% reduction from the mean Taltz baseline score of 38 (SD=17)). Patients receiving placebo achieved a mean NAPSI of 37 (SD=20) at Week 12 (a 2% reduction from the mean placebo baseline score of 39 (SD=17)).8
MMRM=mixed model of repeated measures; NAPSI=Nail Psoriasis Severity Index; SD=standard deviation.
Narrator: In another post-hoc, subgroup analysis of UNCOVER-3, Taltz has also improved nail psoriasis, with a mean improvement from baseline in NAPSI of 39% at Week 12 and 86% at Week 60.
3:00-3:32
[Captions animate to fill the screen.]
Caption:
SPIRIT-P1 and -P2 trial designs1,10-12
SPIRIT-P1, -P2
Participants
SPIRIT-P1 (biologic-naïve N=417), SPIRIT-P2 (TNFi-experienced N=363)*, Patients ≥18 years of page with active PsA, ≥3 swollen and ≥3 tender joints
Trial design
Phase 3, randomized, double-blind, placebo-controlled trials
Dosing
- Taltz 80 mg every 2 weeks† (n=203) (n=123)
- Taltz 80 mg every 4 weeks (n=107) (n=122)
- Placebo (n=106) (n=118)
- SPIRIT-P1 had an active reference arm of Humira® (adalimumab) 40 mg every 2 weeks (n=101)
- Patients randomized to Taltz received a 160 mg starting dose
Primary endpoint
Proportion of patients achieving ACR20 response at Week 24
Patients in all study arms were allowed to continue taking stable background medications during the trial. Inadequate responders (as defined by blinded criteria of <20% improvement in tender and in swollen joint counts) at Week 16 received rescue therapy and were analyzed as nonresponders after week 16 until the primary endpoint. After receiving rescue therapy, inadequate responders in the placebo and Humira arms were re-randomized to Taltz 80 mg every 2 or 4 weeks. NRI methods were used for categorical efficacy analysis during the double-blind treatment period.
*Inadequate response and/or intolerance to 1 or 2 prior TNFis.
†Taltz 80 mg Q2W is not an approved dose to PsA.
ACR20=American College of Rheumatology 20% response rate; NRI=nonresponder imputation; PsA=psoriatic arthritis; TNF-i= TNFi=tumor necrosis factor inhibitor.
Narrator: The designs of the Taltz registration trials of SPIRIT-P1 and SPIRIT-P2 in adults with active psoriatic arthritis are shown here. The primary endpoint of both trials was proportion of patients achieving ACR20 response at Week 24.
Not only can Taltz improve plaque psoriasis, including in challenging body areas like scalp and nail which can be predictors of psoriatic arthritis, but Taltz can also relieve the symptoms of active psoriatic arthritis.
3:33-3:48
[Captions animate to fill the screen.]
Caption:
SPIRIT-P1: Biologic-Naive Patients1,11,13
ACR Response Rates at Week 24, NRI
PATIENTS ACHIEVING RESPONSE (%)
Taltz 80 mg every 4 weeks (n=107)
ACR20, 58%*
ACR50, 40%*
ACR70, 23%*
Placebo (n=106)
ACR20, 30%
ACR50, 15%
ACR70, 6%
*P≤0.001 vs Placebo
SPIRIT-P1: TNFi-Experienced Patients1,11,13
ACR Response Rates at Week 24, NRI
PATIENTS ACHIEVING RESPONSE (%)
Taltz 80 mg every 4 weeks (n=122)
ACR20, 53%†
ACR50, 35%†
ACR70, 22%†
Placebo (n=118)
ACR20, 20%
ACR50, 5%
ACR70, 0%
†P≤0.001 vs Placebo
Primary endpoint=ACR20 response at Week 24.
Inadequate responders (<20% improvement in tender and in swollen joint counts) at Week 16 were analyzed as nonresponders after Week 16 until the primary endpoint.
NRI of intent-to-treat population through Week 24.
ACR20=American College of Rheumatology 20% response rate; ACR50=American College of Rheumatology 50% response rate; ACR70=American College of Rheumatology 70% response rate.
Narrator: In the SPIRIT-P1 and SPIRIT-P2 trials, significantly more patients treated with Taltz achieved ACR20, ACR50, and ACR70 at Week 24, compared to patients treated with placebo.
3:49-4:04
[Drawing of a hand x-ray and captions animate to fill the screen.]
Caption:
FOR BIOLOGIC-NAIVE PATIENTS WITH PSORIATIC ARTHRITIS
67% of patients had no radiographic progression* at Week 156
74% of patients had minimal radiographic progression‡ at Year 3
SPIRIT-P1 (Biologic-Naïve): Adjusted mean change from baseline in mTSS at Week 16, MMRM1,11,13,14
ADJUSTED MEAN CHANGE FROM BASELINE, 0, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6
Taltz 80 mg every 4 weeks (n=107), 0.13†
Humira 40 mg every 2 weeks (n=101), 0.12†
Placebo (n=106), 0.36
mTSS
SPIRIT-P1 was no designed to test the noninferiority or superiority of Taltz against Humira® (adalimumab). Thus, these data should not be used to compare the efficacy between these products.
*Defined as a change from baseline in mTSS≤0; measured in biologic-naive patients (analysis is based on patients with evaluable X-rays at baseline and Year 3).14,15
†P≤0.05 vs placebo. Mean baseline mTSS=19.2 for Taltz, 15.9 for Humira, and 17.6 for placebo.
‡Defined as a change from baseline in mTSS≤0.5; measured in biologic-naive patients (analysis is based on patients with evaluable X-rays at baseline and Year 3).
Missing mTSS data were imputed using NRI and linear extrapolation for Week 16 and Year 3 (Week 156), respectively. In the open-label extension period, linear extrapolation was used if patients had a baseline and at least one post-baseline value (i.e., Week 52, 108 or 156). At Week 156, 10.8% (n=28) were imputed using linear extrapolation.
Inhibition of progression of structural damage was assessed radiographically and expressed as the adjusted mean change in mTSS and its components, the joint space narrowing score and bone erosion score, at Week 16 vs baseline. The mTSS score was modified for PsA by addition of hand distal interphalangeal (DIP) joints.
mTSS is a composite measure found by totaling the joint space narrowing score and the bone erosion score.
SPIRIT-P2 (TNFi-experienced) did not include an assessment of radiographic progression.11
Primary endpoint=ACR20 response at Week 24.
Week 156 mean mTSS change from baseline (linear extrapolation) was 1.7 (Nx=72) for patients entering the extension period and initially randomized Taltz 80 mg every 4 weeks (n=81; mean baseline 19.2).1,14
n=total number of patients entering the open-label extension period population (Week 52-156).13
Nx=number of patients with nonmissing change after linear extrapolation.13
The Week 156 data were from a post hoc analysis and were not placebo-controlled; therefore, statistical conclusions cannot be drawn.
The open-label extension phase of the study has limitations (e.g., no placebo comparison, patients remaining in the extension phase may be those with better results). Patients in the extension period population were all patients administered Taltz on or after Week 24.
ACR20=American College of Rheumatology 20% response rate; DIP=distal interphalangeal; mTSS=modified total Sharp score; NRI=nonresponder imputation; TNFi=tumor necrosis factor inhibitor.
Narrator: Additionally, Taltz demonstrates inhibition of radiographic progression in psoriatic arthritis patients as early as Week 16 and through to 3 years.
4:05-4:32
[Drawings of Taltz prescription box with pen, patient holding their hand, and close-up of hand which appears inflamed appear. Captions then animate to fill the screen.]
Caption:
FOR ADULTS WITH MODERATE-TO-SEVERE PLAQUE PSORIASIS
ADVERSE REACTIONS AND INFECTIONS1 through Week 12
Taltz 80 mg every 2 weeks (n=1167)
Placebo (n=791)
The most frequent injection site reactions were erythema and pain. Most injection site reactions were mild to moderate in severity and did not lead to discontinuation of Taltz.
Adverse reactions that occurred in ≥1% of Taltz patients and more frequently than with placebo
ADVERSE REACTIONS
17%, Injection site reactions, PBO = 3%
14%, Upper respiratory tract infections*, PBO = 13%
2%, Nausea, PBO = 1%
2%, Tinea infections, PBO <1%
*Upper respiratory tract infections include nasopharyngitis and rhinovirus infection.
PBO=placebo.
INFECTIONS
27%, Infections, PBO = 23%
0.4%, Serious infections, PBO = 0.4%
Narrator: Taltz treatment can be accompanied by adverse reactions, the most common being injection site reactions, followed by upper respiratory tract infections, nausea, and tinea infections. Taltz has no boxed warning. Warnings and precautions include infections, pre-treatment evaluation for tuberculosis, hypersensitivity, inflammatory bowel disease, and avoiding live immunizations.
4:33-4:55
[On-screen patient with their arms folded. The Taltz prescription box is pointing to the plaques that are present on the patient’s arms and scalp, as well as their nails. Timeline representing six months appears.]
Caption:
6-month delay
Narrator: Consider Taltz today for your patients with moderate-to-severe plaque psoriasis, especially in challenging body areas. These patients are at a greater risk of developing psoriatic arthritis and even a 6-month diagnostic delay may result in irreversible damage and worsened long-term physical function.
4:56-7:57
[Important Safety Information caption is shown on screen.]
Caption:
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
Taltz is contraindicated in patients with a previous serious hypersensitivity reaction, such as anaphylaxis, to ixekizumab or to any of the excipients.
WARNINGS AND PRECAUTIONS
Infections
Taltz may increase the risk of infection. In clinical trials of adult patients with plaque psoriasis, the Taltz group had a higher rate of infections than the placebo group (27% vs 23%). A similar increase in risk of infection was seen in placebo-controlled trials of adult patients with psoriatic arthritis, ankylosing spondylitis, non-radiographic axial spondyloarthritis, and pediatric patients with plaque psoriasis. Serious infections have occurred. Instruct patients to seek medical advice if signs or symptoms of clinically important chronic or acute infection occur. If a serious infection develops, discontinue Taltz until the infection resolves.
Pre-Treatment Evaluation for Tuberculosis
Evaluate patients for tuberculosis (TB) infection prior to initiating treatment with Taltz. Do not administer to patients with active TB infection. Initiate treatment of latent TB prior to administering Taltz. Closely monitor patients receiving Taltz for signs and symptoms of active TB during and after treatment.
Hypersensitivity
Serious hypersensitivity reactions, including angioedema and urticaria (each ≤0.1%), occurred in the Taltz group in clinical trials. Anaphylaxis, including cases leading to hospitalization, has been reported in post-marketing use with Taltz. If a serious hypersensitivity reaction occurs, discontinue Taltz immediately and initiate appropriate therapy.
Inflammatory Bowel Disease
Patients treated with Taltz may be at an increased risk of inflammatory bowel disease. In clinical trials, Crohn’s disease and ulcerative colitis, including exacerbations, occurred at a greater frequency in the Taltz group than the placebo group. During Taltz treatment, monitor patients for onset or exacerbations of inflammatory bowel disease and if IBD occurs, discontinue Taltz and initiate appropriate medical management.
Immunizations
Prior to initiating therapy with Taltz, consider completion of all age-appropriate immunizations according to current immunization guidelines. Avoid use of live vaccines in patients treated with Taltz.
ADVERSE REACTIONS
Most common adverse reactions (≥1%) associated with Taltz treatment are injection site reactions, upper respiratory tract infections, nausea, and tinea infections. Overall, the safety profiles observed in adult patients with psoriatic arthritis, ankylosing spondylitis, non-radiographic axial spondyloarthritis, and pediatric patients with plaque psoriasis were consistent with the safety profile in adult patients with plaque psoriasis, with the exception of influenza and conjunctivitis in psoriatic arthritis and conjunctivitis, influenza, and urticaria in pediatric psoriasis.
Please see Prescribing Information and Medication Guide. Please see Instructions for Use included with the device.
IX HCP ISI 07MAY2020
Narrator:
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
Taltz is contraindicated in patients with a previous serious hypersensitivity reaction, such as anaphylaxis, to ixekizumab or to any of the excipients.
WARNINGS AND PRECAUTIONS
Infections
Taltz may increase the risk of infection. In clinical trials of adult patients with plaque psoriasis, the Taltz group had a higher rate of infections than the placebo group (27% vs 23%). A similar increase in risk of infection was seen in placebo-controlled trials of adult patients with psoriatic arthritis, ankylosing spondylitis, non-radiographic axial spondyloarthritis, and pediatric patients with plaque psoriasis. Serious infections have occurred. Instruct patients to seek medical advice if signs or symptoms of clinically important chronic or acute infection occur. If a serious infection develops, discontinue Taltz until the infection resolves.
Pre-Treatment Evaluation for Tuberculosis
Evaluate patients for tuberculosis (TB) infection prior to initiating treatment with Taltz. Do not administer to patients with active TB infection. Initiate treatment of latent TB prior to administering Taltz. Closely monitor patients receiving Taltz for signs and symptoms of active TB during and after treatment.
Hypersensitivity
Serious hypersensitivity reactions, including angioedema and urticaria (each ≤0.1%), occurred in the Taltz group in clinical trials. Anaphylaxis, including cases leading to hospitalization, has been reported in post-marketing use with Taltz. If a serious hypersensitivity reaction occurs, discontinue Taltz immediately and initiate appropriate therapy.
Inflammatory Bowel Disease
Patients treated with Taltz may be at an increased risk of inflammatory bowel disease. In clinical trials, Crohn’s disease and ulcerative colitis, including exacerbations, occurred at a greater frequency in the Taltz group than the placebo group. During Taltz treatment, monitor patients for onset or exacerbations of inflammatory bowel disease and if IBD occurs, discontinue Taltz and initiate appropriate medical management.
Immunizations
Prior to initiating therapy with Taltz, consider completion of all age-appropriate immunizations according to current immunization guidelines. Avoid use of live vaccines in patients treated with Taltz.
ADVERSE REACTIONS
Most common adverse reactions (≥1%) associated with Taltz treatment are injection site reactions, upper respiratory tract infections, nausea, and tinea infections. Overall, the safety profiles observed in adult patients with psoriatic arthritis, ankylosing spondylitis, non-radiographic axial spondyloarthritis, and pediatric patients with plaque psoriasis were consistent with the safety profile in adult patients with plaque psoriasis, with the exception of influenza and conjunctivitis in psoriatic arthritis and conjunctivitis, influenza, and urticaria in pediatric psoriasis.
Please see Prescribing Information and Medication Guide. Please see Instructions for Use included with the device.
7:58-8:09
[Reference list progresses on screen. Text fades to white. Lilly logo appears.]
Caption:
References
- Taltz. Prescribing Information. Lilly USA, LLC.
- Wilson FC, Icen M, Crowson CS, McEvoy MT, Gabriel SE, Kremers HM. Incidence and clinical predictors of psoriatic arthritis in patients with psoriasis: a population-based study. Arthritis Rheum. 2009;61(2):233-239.
- Prey S, Paul C, Bronsard V, et al. Assessment of risk of psoriatic arthritis in patients with plaque psoriasis: a systematic review of the literature. J Eur Acad Dermatol Venereol. 2010;24(suppl 2):31-35. doi:10.1111/j.1468-3083.2009.03565.x
- Gelfand JM, Gladman DD, Mease PJ, et al. Epidemiology of psoriatic arthritis in the population of the United States. J Am Acad Dermatol. 2005;53(4):573-577.
- Data on file. Lilly USA, LLC. DOF-IX-US-0183.
- Data on file. Lilly USA, LLC. DOF-IX-US-0008.
- Reich K, Leonardi C, Lebwohl M, et al. Sustained response with ixekizumab treatment of moderate-to-severe psoriasis with scalp involvement: results from three phase 3 trials (UNCOVER-1, UNCOVER-2, UNCOVER-3). J Dermatolog Treat. 2017;28:282-287.
- Data on file. Lilly USA, LLC. TAL20170829A.
- Dennehy EB, Zhang L, Amato D, Goldblum O, Rich P. Ixekizumab is effective in subjects with moderate to severe plaque psoriasis with significant nail involvement: results from UNCOVER 3. J Drugs Dermatol. 2016;15:958-961.
- Mease PJ, van der Heijde D, Ritchlin CT, et al; on behalf of SPIRIT-P1 Study Group. Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase 3 trial SPIRIT-P1. Ann Rheum Dis. 2017;76(suppl):1-30.
- Nash P, Kirkham B, Okada M, et al; on behalf of SPIRIT-P2 Study Group. Ixekizumab for the treatment of patients with active psoriatic arthritis and an inadequate response to tumour necrosis factor inhibitors: results from the 24-week randomised, double-blind, placebo-controlled period of the SPIRIT-P2 phase 3 trial. Lancet. 2017;389:2317-2327.
- Nash P, Kirkham B, Okada M, et al; on behalf of SPIRIT-P2 Study Group. Ixekizumab for the treatment of patients with active psoriatic arthritis and an inadequate response to tumour necrosis factor inhibitors: results from the 24-week randomised, double-blind, placebo-controlled period of the SPIRIT-P2 phase 3 trial. Lancet. 2017;389:2317-2327. Supplementary appendix.
- Mease PJ, van der Heijde D, Ritchlin CT, et al; on behalf of SPIRIT-P1 Study Group. Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase 3 trial SPIRIT-P1. Ann Rheum Dis. 2017;76:79-87.
- Data on file. Lilly USA, LLC. DOF-IX-US-0094.
- Chandran V, van der Heijde D, Fleischmann RM, et al. Ixekizumab treatment of biologic-naïve patients with active psoriatic arthritis: 3-year results from a phase III clinical trial (SPIRIT-P1). Rheumatology (Oxford). 2020;59:2774-2784.
For more information, visit Taltz.com/HCP
Lilly
PP-IX-US-6493 01/2024 ©Lilly USA 2024. All rights reserved.
Narrator: For more information, visit Taltz.com/HCP